women-owned,
purpose driven
Quality exam gloves dedicated to empowering women in business
Inclusivity
Opportunities for women, across industries, worldwide.
Innovation
Forward-thinking solutions ensure consistency you can count on.
Quality
Rigorous testing to ensure top-tier end product.
Pioneered for possibilities,
powered by progress.
When you choose SYNA Medical, you're not just purchasing top-quality gloves — you're supporting a global network of women entrepreneurs, researchers, and academics.
It's the SYNA Phenomenon
Since SYNA supports women-led initiatives, your purchase has a ripple effect, opening doors for the next generation of women entrepreneurs. Choosing SYNA means embracing inclusivity. It means your daughter, sister, or friend will be more easily recognized, valued, and celebrated for their contributions, building a brighter future for women everywhere.
The Women that Power SYNA
Co-founders Hindy Rosenberg and Anita Lovse established SYNA with two passions; to empower women entrepreneurs, and to provide reliable, quality healthcare supplies where they are most needed.


Endorsed by
WBENC
With decades of combined experience in branding, marketing, and medical supply distribution, Hindy and Anita have taken a leading role in national-level initiatives in the healthcare sector, earning them certification by the Women’s Business Enterprise National Council (WBENC).
"We're delighted to be working with Medline and a women-owned business like SYNA Medical where our supplier diversity goals are matched perfectly with their outstanding product quality and expertise."
David S. Harrington, Executive Chairman at Genesis HealthCare
Nitrile and Vinyl Exam Gloves:
Both provide clinical-grade protection
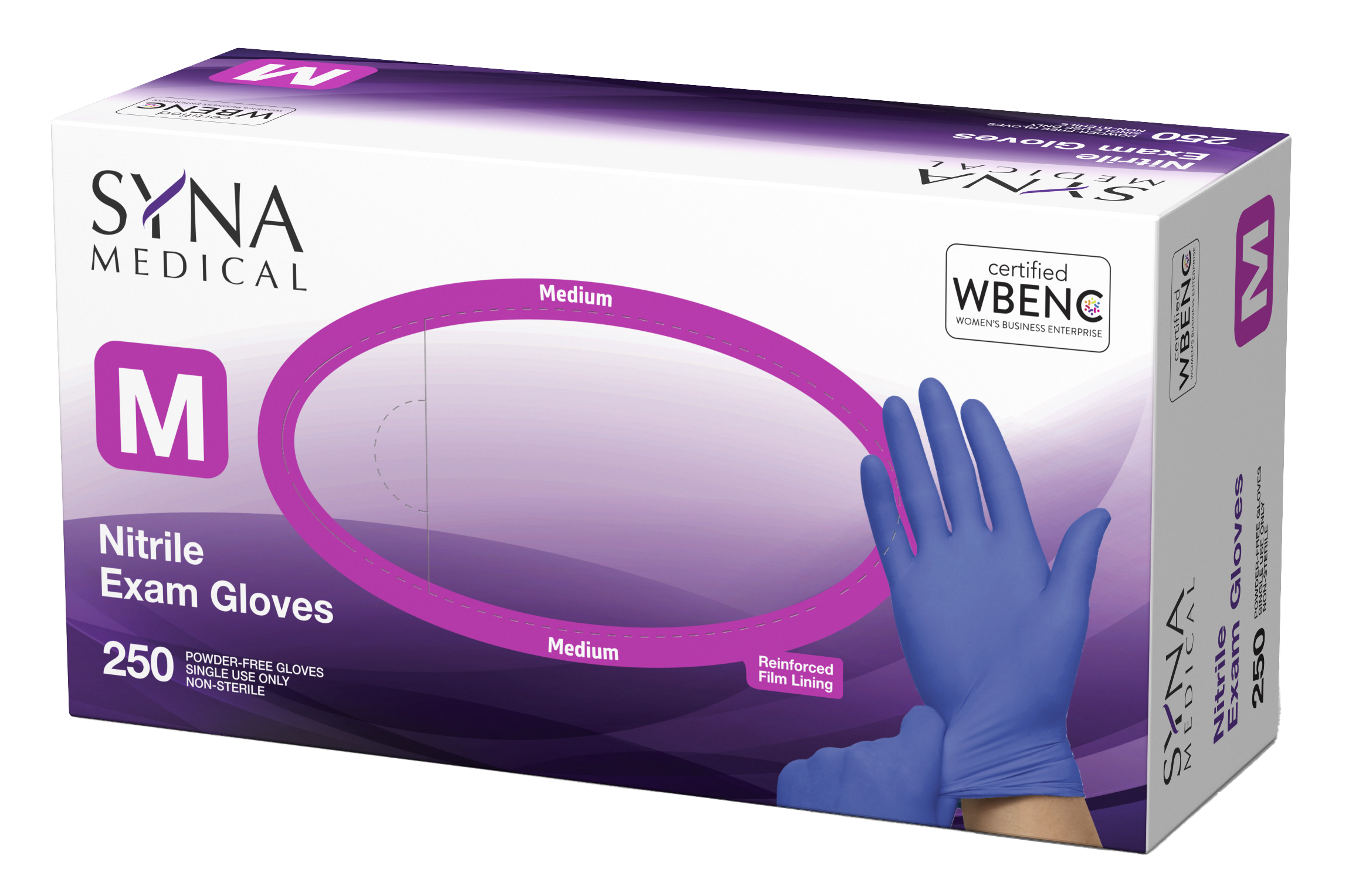
- Manufactured under supervision in high-speed, double-track production lines
- Meets and exceeds ASTM D6319, ASTM D6978, ASTM F1671 standards and certification
- Each batch is thoroughly inspected, subject to SGS Final Random Inspection AQL 1.5
- Additional samples are sent for Lab testing to confirm chemical formulation based on +95% Acrylonitrile Butadiene Rubber
We're proud to partner
with Medline
SYNA's gloves are distributed exclusively via Medline, a global leader in healthcare supplies. When you choose SYNA Medical, you also choose the exceptional service and resources that Medline can offer. At the core of this arrangement are the shared values that elevate the SYNA and Medline relationship to that of a dream team.